The clustered often interspaced brief palindromic repeat (CRISPR) know-how holds the promise to revolutionize gene modifying applied sciences, which is transformative to the best way we perceive and deal with illnesses. This method relies in a pure mechanism present in micro organism that permits a protein coupled to a single information RNA (gRNA) strand to find and make cuts in particular websites within the focused genome. Having the ability to computationally predict the effectivity and specificity of gRNA is central to the success of gene modifying.
Transcribed from DNA sequences, RNA is a vital kind of organic sequence of ribonucleotides (A, U, G, C), which folds into 3D construction. Benefiting from current advance in massive language fashions (LLMs), quite a lot of computational biology duties might be solved by fine-tuning organic LLMs pre-trained on billions of identified organic sequences. The downstream duties on RNAs are comparatively understudied.
On this publish, we undertake a pre-trained genomic LLMs for gRNA effectivity prediction. The concept is to deal with a pc designed gRNA as a sentence, and fine-tune the LLM to carry out sentence-level regression duties analogous to sentiment evaluation. We used Parameter-Environment friendly Nice-Tuning strategies to cut back the variety of parameters and GPU utilization for this activity.
Resolution overview
Massive language fashions (LLMs) have gained a number of curiosity for his or her potential to encode syntax and semantics of pure languages. The neural structure behind LLMs are transformers, that are comprised of attention-based encoder-decoder blocks that generate an inner illustration of the information they’re educated from (encoder) and are capable of generate sequences in the identical latent area that resemble the unique information (decoder). Because of their success in pure language, current works have explored the usage of LLMs for molecular biology info, which is sequential in nature.
DNABERT is a pre-trained transformer mannequin with non-overlapping human DNA sequence information. The spine is a BERT structure made up of 12 encoding layers. The authors of this mannequin report that DNABERT is ready to seize a very good function illustration of the human genome that permits state-of-the-art efficiency on downstream duties like promoter prediction and splice/binding web site identification. We determined to make use of this mannequin as the muse for our experiments.
Regardless of the success and widespread adoption of LLMs, fine-tuning these fashions might be tough due to the variety of parameters and computation vital for it. For that reason, Parameter-Environment friendly Nice-Tuning (PEFT) strategies have been developed. On this publish, we use one in all these strategies, known as LoRA (Low-Rank Adaptation). We introduce the tactic within the following sections.
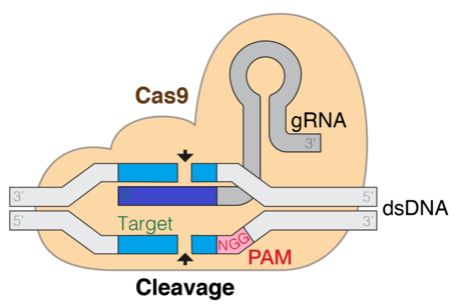
The next diagram is a illustration of the Cas9 DNA goal mechanism. The gRNA is the part that helps goal the cleavage web site.

The aim of this answer is to fine-tune a base DNABERT mannequin to foretell exercise effectivity from completely different gRNA candidates. As such, our answer first takes gRNA information and processes it, as described later on this publish. Then we use an Amazon SageMaker pocket book and the Hugging Face PEFT library to fine-tune the DNABERT mannequin with the processed RNA information. The label we need to predict is the effectivity rating because it was calculated in experimental situations testing with the precise RNA sequences in cell cultures. These scores describe a stability between having the ability to edit the genome and never harm DNA that wasn’t focused.
The next diagram illustrates the workflow of the proposed answer.

Conditions
For this answer, you want entry to the next:
- A SageMaker pocket book occasion (we educated the mannequin on an ml.g4dn.8xlarge occasion with a single NVIDIA T4 GPU)
- transformers-4.34.1
- peft-0.5.0
- DNABERT 6
Dataset
For this publish, we use the gRNA information launched by researchers in a paper about gRNA prediction utilizing deep studying. This dataset incorporates effectivity scores calculated for various gRNAs. On this part, we describe the method we adopted to create the coaching and analysis datasets for this activity.
To coach the mannequin, you want a 30-mer gRNA sequence and effectivity rating. A k-mer is a contiguous sequence of okay nucleotide bases extracted from an extended DNA or RNA sequence. For instance, if in case you have the DNA sequence “ATCGATCG” and also you select okay = 3, then the k-mers inside this sequence can be “ATC,” “TCG,” “CGA,” “GAT,” and “ATC.”
Effectivity rating
Begin with excel file 41467_2021_23576_MOESM4_ESM.xlsx from the CRISPRon paper within the Supplementary Knowledge 1 part. On this file, the authors launched the gRNA (20-mer) sequences and corresponding total_indel_eff scores. We particularly used the information from the sheet named spCas9_eff_D10+dox. We use the total_indel_eff column because the effectivity rating.
Coaching and validation information
Given the 20-mers and the crispron scores (identical because the total_indel_eff scores) from earlier, full the next steps to place collectively the coaching and validation information:
- Convert the sequences within the sheet “TRAP12K microarray oligos” into an .fa (fasta) file.
- Run the script
get_30mers_from_fa.py(from the CRISPRon GitHub repository) to acquire all doable 23-mers and 30-mers from the sequences obtained from Step 1. - Use the
CRISPRspec_CRISPRoff_pipeline.pyscript (from the CRISPRon GitHub repository) to acquire the binding power for the 23-mers obtained from Step 2. For extra particulars on run this script, try the code launched by the authors of the CRISPRon paper(examine the scriptCRISPRon.sh). - At this level, we now have 23-mers together with the corresponding binding power scores, and 20-mers together with the corresponding CRISPRon scores. Moreover, we now have the 30-mers from Step 2.
- Use the script
prepare_train_dev_data.py(from our launched code) to create coaching and validation splits. Working this script will create two information:practice.csvanddev.csv.
The information appears one thing like the next:
Mannequin structure for gRNA encoding
To encode the gRNA sequence, we used the DNABERT encoder. DNABERT was pre-trained on human genomic information, so it’s a very good mannequin to encode gRNA sequences. DNABERT tokenizes the nucleotide sequence into overlapping k-mers, and every k-mer serves as a phrase within the DNABERT mannequin’s vocabulary. The gRNA sequence is damaged right into a sequence of k-mers, after which every k-mer is changed by an embedding for the k-mer on the enter layer. In any other case, the structure of DNABERT is much like that of BERT. After we encode the gRNA, we use the illustration of the [CLS] token as the ultimate encoding of the gRNA sequence. To foretell the effectivity rating, we use a further regression layer. The MSE loss would be the coaching goal. The next is a code snippet of the DNABertForSequenceClassification mannequin:
Nice-tuning and prompting genomic LLMs
Nice-tuning all of the parameters of a mannequin is pricey as a result of the pre-trained mannequin turns into a lot bigger. LoRA is an progressive method developed to handle the problem of fine-tuning extraordinarily massive language fashions. LoRA gives an answer by suggesting that the pre-trained mannequin’s weights stay fastened whereas introducing trainable layers (known as rank-decomposition matrices) inside every transformer block. This method considerably reduces the variety of parameters that have to be educated and lowers the GPU reminiscence necessities, as a result of most mannequin weights don’t require gradient computations.
Subsequently, we adopted LoRA as a PEFT methodology on the DNABERT mannequin. LoRA is applied within the Hugging Face PEFT library. When utilizing PEFT to coach a mannequin with LoRA, the hyperparameters of the low rank adaptation course of and the best way to wrap base transformers fashions might be outlined as follows:
Maintain-out analysis performances
We use RMSE, MSE, and MAE as analysis metrics, and we examined with rank 8 and 16. Moreover, we applied a easy fine-tuning methodology, which is solely including a number of dense layers after the DNABERT embeddings. The next desk summarizes the outcomes.
| Technique | RMSE | MSE | MAE |
| LoRA (rank = 8) | 11.933 | 142.397 | 7.014 |
| LoRA (rank = 16) | 13.039 | 170.01 | 7.157 |
| One dense layer | 15.435 | 238.265 | 9.351 |
| Three dense layer | 15.435 | 238.241 | 9.505 |
| CRISPRon | 11.788 | 138.971 | 7.134 |
When rank=8, we now have 296,450 trainable parameters, which is about 33% trainable of the entire. The efficiency metrics are “rmse”: 11.933, “mse”: 142.397, “mae”: 7.014.
When rank=16, we now have 591,362 trainable parameters, which is about 66% trainable of the entire. The efficiency metrics are “rmse”: 13.039, “mse”: 170.010, “mae”: 7.157. There may need some overfitting problem right here beneath this setting.
We additionally examine what occurs when including a number of dense layers:
- After including one dense layer, we now have “rmse”: 15.435, “mse”: 238.265, “mae”: 9.351
- After including three dense layers, we now have “rmse”: 15.435, “mse”: 238.241, “mae”: 9.505
Lastly, we examine with the prevailing CRISPRon methodology. CRISPRon is a CNN primarily based deep studying mannequin. The efficiency metrics are “rmse”: 11.788, “mse”: 138.971, “mae”: 7.134.
As anticipated, LoRA is doing significantly better than merely including a number of dense layers. Though the efficiency of LoRA is a bit worse than CRISPRon, with thorough hyperparameter search, it’s prone to outperform CRISPRon.
When utilizing SageMaker notebooks, you could have the pliability to save lots of the work and information produced throughout the coaching, flip off the occasion, and switch it again on while you’re able to proceed the work, with out shedding any artifacts. Turning off the occasion will maintain you from incurring prices on compute you’re not utilizing. We extremely advocate solely turning it on while you’re actively utilizing it.
Conclusion
On this publish, we confirmed use PEFT strategies for fine-tuning DNA language fashions utilizing SageMaker. We centered on predicting effectivity of CRISPR-Cas9 RNA sequences for his or her impression in present gene-editing applied sciences. We additionally offered code that may enable you jumpstart your biology functions in AWS.
To study extra in regards to the healthcare and life science area, consult with Run AlphaFold v2.0 on Amazon EC2 or fine-tuning Nice-tune and deploy the ProtBERT mannequin for protein classification utilizing Amazon SageMaker.
In regards to the Authors
 Siddharth Varia is an utilized scientist in AWS Bedrock. He’s broadly desirous about pure language processing and has contributed to AWS merchandise comparable to Amazon Comprehend. Exterior of labor, he enjoys exploring new locations and studying. He bought on this challenge after studying the ebook The Code Breaker.
Siddharth Varia is an utilized scientist in AWS Bedrock. He’s broadly desirous about pure language processing and has contributed to AWS merchandise comparable to Amazon Comprehend. Exterior of labor, he enjoys exploring new locations and studying. He bought on this challenge after studying the ebook The Code Breaker.
 Yudi Zhang is an Utilized Scientist at AWS advertising and marketing. Her analysis pursuits are within the space of graph neural networks, pure language processing, and statistics.
Yudi Zhang is an Utilized Scientist at AWS advertising and marketing. Her analysis pursuits are within the space of graph neural networks, pure language processing, and statistics.
 Erika Pelaez Coyotl is a Sr Utilized Scientist in Amazon Bedrock, the place she’s at present serving to develop the Amazon Titan massive language mannequin. Her background is in biomedical science, and she or he has helped a number of prospects develop ML fashions on this vertical.
Erika Pelaez Coyotl is a Sr Utilized Scientist in Amazon Bedrock, the place she’s at present serving to develop the Amazon Titan massive language mannequin. Her background is in biomedical science, and she or he has helped a number of prospects develop ML fashions on this vertical.
 Zichen Wang is a Sr Utilized Scientist in AWS AI Analysis & Schooling. He’s desirous about researching graph neural networks and making use of AI to speed up scientific discovery, particularly on molecules and simulations.
Zichen Wang is a Sr Utilized Scientist in AWS AI Analysis & Schooling. He’s desirous about researching graph neural networks and making use of AI to speed up scientific discovery, particularly on molecules and simulations.
 Rishita Anubhai is a Principal Utilized Scientist in Amazon Bedrock. She has deep experience in pure language processing and has contributed to AWS initiatives like Amazon Comprehend, Machine Studying Options Lab, and growth of Amazon Titan fashions. She’s keenly desirous about utilizing machine studying analysis, particularly deep studying, to create tangible impression.
Rishita Anubhai is a Principal Utilized Scientist in Amazon Bedrock. She has deep experience in pure language processing and has contributed to AWS initiatives like Amazon Comprehend, Machine Studying Options Lab, and growth of Amazon Titan fashions. She’s keenly desirous about utilizing machine studying analysis, particularly deep studying, to create tangible impression.